
Triad Japan is a Site Network with strong expertise and a proven track record in the CNS (Central Nervous System) field, particularly in psychiatric disorders.
We support clinical trials and clinical research across all therapeutic areas, ensuring studies are conducted properly and smoothly.
Drawing on the know-how gained through new drug development, we strive to help address today’s medical challenges.
Message from the Head
Advancing Innovative Healthcare Together
The landscape of clinical trials is undergoing rapid and transformative change.
Since our founding in 2000, our team—grounded in deep expertise in the CNS (Central Nervous System) field—has supported trial sites through CRC (Clinical Research Coordinator) Services, Clinical Trial Administrative Services, and a range of specialized solutions. While psychiatric and neurological disorders remain our core strength, we have steadily expanded our capabilities to support clinical trials across a broad spectrum of therapeutic areas.
In the CNS field in particular, we deliver not only robust Site Network functions but also highly specialized services such as psychological rater dispatch performed by licensed clinical psychologists. In addition, our patient recruitment services extend across multiple therapeutic areas, enabling us to support diverse studies with flexibility, efficiency, and speed.
As new models of clinical trials emerge—driven by GCP Renovation, digital innovation, and increasing global collaboration—Site Networks must continue to evolve and redefine their value. Guided by our Triad Values of Teamwork, Responsibility, Integrity, Agility, and Diversity, we embrace change and work closely with partners around the world to create meaningful impact in clinical development.
Each member of our division is committed to strengthening their professional expertise, anticipating future needs, and taking on new challenges with agility. As a forward-looking Site Network & Clinical Support Services Division, we will continue to evolve, deliver new value, and contribute to the advancement of innovative healthcare in Japan and beyond.
We sincerely appreciate your continued partnership and support.
Miho Goto
Executive Vice President & Representative Director
Head of the Site Network & Clinical Support Services Division
Business Overview
As a Site Network, we provide comprehensive support for clinical trials, including trial operations, trial-related administrative services, and IRB (Institutional Review Board) administration.
Working with clinics and large hospitals mainly in Tokyo and Kanagawa, we offer a support structure that covers all therapeutic areas—from CNS to infectious diseases—and accommodates a wide range of studies, from Phase I to Phase IV and clinical research.
Our skilled CRC (Clinical Research Coordinator) and Clinical Trial Administrative Services teams support trial sites to ensure that studies are conducted properly and smoothly, in compliance with GCP and all relevant regulations.
Clinical Trial Support Track Record
In the CNS field, advances in digital technology are enabling the development of innovative new drugs and medical devices.We are actively embracing these emerging areas and are steadily building our track record without pause.
CNS Unit Track Record: Number of Protocols


As of September 2025
General Unit Track Record: Number of Protocols

As of September 2025
Full Support
from Study Start-Up to Execution and Completion
The Site Network & Clinical Support Services Division provides CRC Services, Clinical Trial Administrative Services, and IRB Administrative Services, offering comprehensive support from study start-up through conduct and close-out.
CRC Services
-
Coordinating communication among investigators, nurses, participants, and sponsors
-
Checking participant information and managing study schedules
-
Ensuring protocol compliance
-
Assisting with informed consent
-
Explaining investigational product handling
-
Supporting adverse event management
-
And other related tasks
Clinical Trial Administrative Services
-
Preparing required regulatory documents
-
Managing document storage
-
Drafting various clinical trial–related contracts
-
Supporting contract execution
-
Assisting with payments
-
Support for payments,
-
And other related administrative tasks
IRB Administrative Services
-
Preparing for IRB meetings
-
Drafting IRB meeting minutes
-
Preparing required regulatory documents
-
Managing document storage
-
And other related administrative tasks
Triad Japan’s Strengths
A Tailored Support Structure Optimized for Each Project
CRC Services
Our CRC team consists of two specialized units: the CNS Unit, staffed with CRCs who possess deep knowledge, expertise, and patient-care skills in psychiatric disorders; and the General Unit, staffed with CRCs who are qualified nurses, pharmacists, clinical laboratory technologists, and other professionals with broad medical knowledge and experience.
For each project, we rapidly establish an optimal CRC Services structure and contribute to the high-quality execution of clinical trials.
Clinical Trial Administrative Services
We provide comprehensive support for establishing Clinical Trial/Clinical Research Administration Offices, Institutional Review Boards (IRBs), Research Ethics Committees (RECs), and Japan’s Certified Review Boards (CRBs).Our services cover the preparation of documents required by GCP, life science and medical research guidelines, and the Clinical Trials Act, ensuring a compliant environment for clinical trials, clinical research, and specified clinical research.
We also support the digitalization and paperless management of documents and have implemented remote SDV systems (remote Source Data Verification systems).
In addition, we offer Central IRB Administrative Services and have extensive experience supporting Central IRBs, particularly in the CNS field.
Psychological Rater Dispatch Services
In clinical trials, particularly in psychiatric and Alzheimer’s disease studies, psychological assessments and related support play a crucial role. We dispatch licensed clinical psychologists to trial sites to conduct psychological assessments on behalf of investigators or in-house psychologists.
By engaging external psychological raters, sponsors and sites can enhance trial efficiency, reduce bias in efficacy evaluations, and minimize variability in psychological assessment outcomes.
Quality Management
We ensure high-quality clinical trial operations by standardizing procedures through SOPs and manuals and by maintaining strict compliance with GCP and relevant regulations. We also enhance the quality of trial-related documents and records and conduct Quality Management appropriately.
(Japan Association of Site Management Organizations — J-Audit Compliance: April 27, 2017 )


We conduct QMS activities on two fronts: organization-wide efforts to gather information and drive improvements, and daily improvements at the project level within our CRC and Clinical Trial Administrative Services teams.
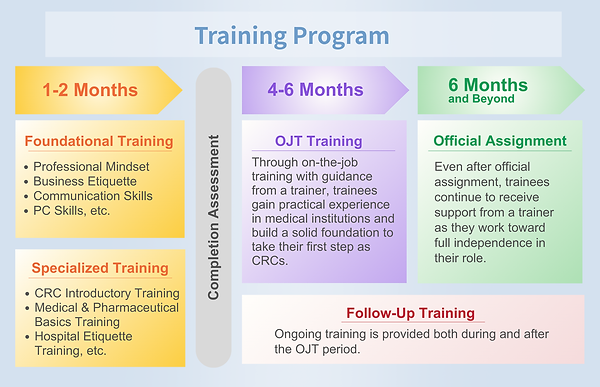
Training & Education
We focus on developing CRCs with strong expertise in medicine, pharmacology, and GCP-related regulations, as well as high ethical standards and patient-centered communication skills. We also provide specialized training programs to cultivate CRCs with advanced competency in the CNS field.
.png)
Code of Conduct
We comply with GCP, all relevant regulations, social standards, and corporate ethics. With a strong commitment to ethics and compliance, we conduct our operations with the highest priority placed on the safety and well-being of study participants.
Mission
Mission
Creating Better Health for All through Total Medical Innovation (“Soui-Soken").
Vision
Vision
Driving Medical Innovation at the Frontlines of Clinical Care.
Value
Value
Every Decision, Driven by What Advances Patients and Medicine.

Quality Policy
Quality Policy
We set quality goals aligned with customer needs, build and refine optimal processes, and act with responsibility and integrity to deliver our best.
Corporate History
Since launching our Site Network business in 2000, we have partnered with Dr. Mitsukuni Murasaki, Director of the CNS Pharmacology Institute, and Dr. Jun Ishigooka, Senior Researcher, to provide comprehensive support for clinical trials at medical institutions, ensuring that trials are conducted appropriately and efficiently.
2000 Jul
Opening of MedSquare Co., Ltd. CNS Pharmacology Institute Kanagawa Office
2007 Jul
Opening of Tokyo Branch
2008 Feb
Business integration from MedSquare Co., Ltd. to Triad Japan Co., Ltd.
2009 Mar
Opening of Nagoya Office and Ogura Office
2012 May
Closure of CNS Pharmacology Institute Ogura Office
2012 Oct
Closure of CNS Pharmacology Institute Nagoya Office
2014 Sep
Tokyo Branch relocated to Yoyogi
2019 Jul
Business alliance with Satt Co., Ltd. based on capital partnership
Compliance Policy of the Site Network & Clinical Support Services Division
The Site Network & Clinical Support Services Division conducts all operations with integrity and in full compliance with applicable laws and regulations. Our activities are guided by the following principles:
1. Respect for Human Life Ensure the scientific, ethical, and objective conduct of clinical trials, with the highest priority placed on the rights, safety, and welfare of study participants. 2. Compliance with Laws and Regulations Comply with GCP (Ministerial Ordinance No. 28) and all other relevant laws, ordinances, and regulations in all clinical trial support and related activities. 3. Compliance with the Antimonopoly Act Do not engage in private monopolization, cartels, or unfair trade practices. 4. Prohibition of Unfair Competition Refrain from improperly acquiring or using trade secrets or other confidential information. 5. Prohibition of Bribery Do not offer or provide improper benefits to government officials, public hospital personnel, or business partners. 6. Avoidance of Conflicts of Interest Perform duties honestly and do not engage in conduct contrary to the company’s interests. 7. Proper Information Management Protect study participant information, confidential data, and personal information, and prevent unauthorized disclosure. 8. No Involvement with Antisocial Forces Maintain a strict stance against antisocial groups and refrain from any association or provision of benefits.
Information Security Policy
Triad Japan Co., Ltd. is committed to ensuring the trust and satisfaction of our customers and all individuals involved in our services, aiming to be a reliable and secure organization.
Code of Conduct 1. Protect information assets by implementing appropriate organizational and technical measures, and respond to evolving technologies and threats. 2. Provide information security training to all employees and ensure thorough awareness of this policy. 3. Set and review information security objectives regularly, and maintain continuous improvement. 4. Assign responsibility and authority to a management representative to execute and improve the management system. June 15, 2022 Triad Japan Co., Ltd. Miho Goto Executive Vice President & Representative Director/ Head of the Site Network & Clinical Support Services Division



